Abstract
Background: SARS-CoV-2 related morbidity and mortality have decreased dramatically since the introduction of mass vaccination programmes. Due to emergence of new variants and waning immunity post-vaccination, multiple countries offer repeated vaccinations to vulnerable patients. Several studies have reported immune thrombocytopaenia (ITP) relapse following SARS-CoV-2 vaccine. We aimed to explore how effective SARS-CoV-2 vaccination is in patients with ITP and how post-vaccine relapse rate compares to the expected monthly ITP relapse rate.
Method: Data from two tertiary centres, Imperial College Healthcare NHS Trust London (ICL), and Erasmus University Medical Centre Rotterdam (EMC), were merged to assess relapse rate and antibody response following SARS-CoV-2 vaccination in patients with ITP aged 14 and over. Patients who received at least one of the first three SARS-CoV-2 vaccine doses and who had one platelet count recorded within 30 days following a dose were included. Relapse was defined within 30 days following vaccination, as either a drop in platelet >50% with a nadir count <30x109/L or a drop in platelets <50% with a nadir count <30x109/L associated with a new bleeding event. All patients who were also under follow up in 2019 were included in a baseline study cohort to determine baseline relapse rate; this was calculated as relapse within 30 days following the first clinic appointment in 2019. Antibody titres obtained 14 to 30 days following vaccination were analysed using Abbot Alinity analyser at ICL and Liaison SARS-CoV-2 TrimericS IgG assay at EMC; both laboratories provided definitions of clinical antibody response (titre > 568 BAU/ml and > 300 BAU/ml respectively). Patients who received IVIGs within 3 months were excluded from this analysis.
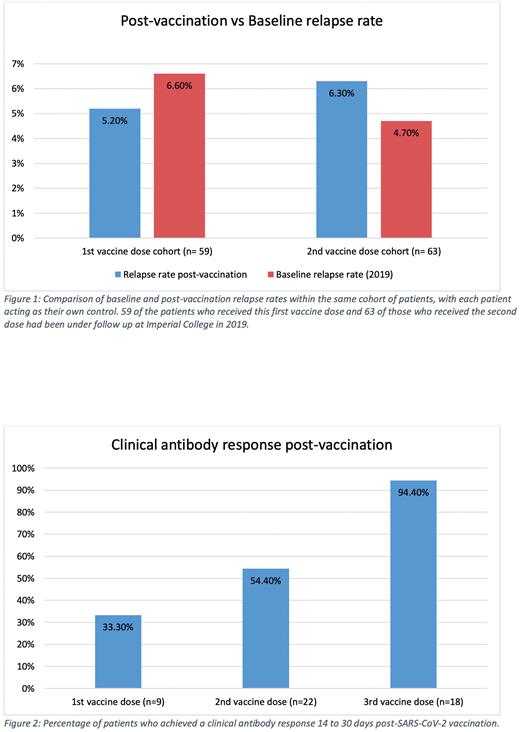
Results: 124 patients were included in the study. The baseline monthly relapse rate of the patients under follow-up in 2019 was calculated as 7.6%.
117 patients received the first SARS-CoV-2 vaccine dose; 47% had Pfizer-BioNTech, 30% ChAdOx1 nCoV-19 and 18% Moderna mRNA-1273. Median platelet count was 116 x109/L (IQR 69-185) before and 120 x109/L (IQR 68-174) after the first vaccine. 5 patients (4.2%) relapsed following vaccination; their median platelet count was 31x109/L before and 13x109/L after; 3 developed bleeding symptoms and received rescue therapy. Of 9 patients tested, 3 (33%) had a clinical antibody response following the first vaccine; none of these patients had received rituximab in the preceding 12 months.
Of 109 patients who received the second SARS-CoV-2 vaccine, 47% had Pfizer-BioNTech, 31% ChAdOx1 nCoV-19 and 18% Moderna mRNA-1273. The median platelet count was 124x109/L (IQR 65-193) pre- and 110 x109/L (IQR 56-185) post-vaccination. Ten patients (9.1%) relapsed following vaccination; 6 experienced bleeding symptoms and received rescue therapy or modifications of long-term therapy. Their median platelet count was 63 x109/L (IQR 28-105) before and 18 x109/L (IQR 11-26) after. Of 22 patients who had an antibody titre recorded post-second vaccination, 12 (54.4%) had a clinical antibody response. Patients who were on steroids, received rituximab in the previous 12 months or had had a splenectomy did not develop a clinical antibody response. 79% of patients who received Pfizer-BioNTech developed a clinical antibody response compared to 14% of those who received ChAdOx1 nCoV-19.
Of 67 patients receiving a third SARS-CoV-2 vaccine dose, 43% received Pfizer, 15% Moderna mRNA-1273 and 42% either Moderna mRNA-1273 or Pfizer-BioNTech (not specifically recorded). Median platelet count was 118 x109/L (IQR 75-201) before and 110 x109/L (IQR 60-194) after. 2 patients (2.9%) relapsed following vaccination; there were no bleeding complications but both received rescue treatment. 17/18 patients achieved a clinical antibody response, including those on steroids and who had undergone splenectomy. The one patient who did not respond had received rituximab 3 months before.
Conclusions: The rate of ITP relapse following SARS-CoV-2 vaccination was not significantly different to the baseline relapse rate in 2019 (Figure 1). No major bleeding events occurred within 30 days of vaccination. Apart from those receiving rituximab, all patients had a clinical antibody response following three vaccine doses (Figure 2). Overall, SARS-CoV-2 vaccination appears to be safe and effective in patients with ITP however 3 vaccine doses may be required.
Disclosures
Bussel:Rallybio: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: Data and Safety Monitoring Board; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; UCB: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data and Safety Monitoring Board; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Jansen:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Research Funding; Principia: Research Funding; Argenx: Research Funding. Cooper:Sanofi, Principia, Novartis, Griffols, Sobi, Argenyx, UCB, Rigel: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal